|
The primary mission of the facility is in line with the NSF grant and Chicago State
University mission with the major goal of supporting research, teaching, curriculum
improvement, and professional development in the Department of Biological Sciences
at Chicago State University. The FACS system allows the users to identify and sort
subpopulations of cells based on natural or experimentally introduced parameters that
include cell-surface molecules, genetic content, and general cell shape and size.
Supplementary microscopy is also available in the director's lab and in the Department
of Biological sciences.
Facility goals
- To undertake the four grant projects proposed for the NSF grant award which entails
performing the necessary sorting of mammalian gut mucosa and immune cells (Drs Walid
Al-Ghoul and Kevin Swier) protozoa (Dr Andrew Maselli), and plant protoplasts (Dr
Devi Poturi) for the purpose of determining cellular mechanisms associated with the
intestinal mucosa barrier, host-pathogen interactions, cell vesicle traffic, and environmental
conservation and crop improvement.
- To disseminate the research findings and knowledge using the flow cytometry facility
by faculty and their students, thus assuring that the acquisition of a FACSaria instrument
will have a major impact on the research exploration and training environment at Chicago
State University, bringing it to a level that is more compatible with current standards
in research and training.
- To enhance Chicago State University's infrastructure needed for teaching, curriculum
improvement, and professional development. Thus, the facility is expected to play
a central role in expanding inquiry-based learning, fostering student collaborations,
and providing valuable hands-on experience with advanced technology. This is certain
to advance the level and quality of instruction in courses taught or designed by the
PI and co-PI's which span the fields of cell biology, parasitology, immunology, physiology,
pathology, botany, and microbiology.
- To design hands-on workshops and training grants that are also expected to benefit
from the FACS system.
- To support Chicago State University mission, and its growing number of programs aimed
at enhancing underrepresented minority participation in basic science research and
channeling B.Sc. and M.Sc. graduates into professional degree-granting institutions.
These programs include the NIH minority biomedical research support (MBRS) and the
NSF alliance for minority participation (AMP) supported research training.
- Ultimately, the FACS facility is expected to contribute to improved recruitment and
empowerment of minority students through academic and research training that incorporates
up-to-date scientific and biomedical technology. This, in turn, will contribute to
increased diversity in the American landscape of skilled professionals.
Sources of Support
This facility was made possible by a generous grant from the National Science Foundation
entitled: "Acquisition Of A Fluorescence-Activated Cell Sorter To Support Research
And Research Training at Chicago State University, A Minority Serving, Undergraduate
Institution". In addition to this grant award with Dr Walid Al-Ghoul as the principal
investigator, the space, infrastructures and ongoing funding to run the facility is
provided by the university administration with strong support from the then University
President, Elnora Daniel, the Dean of College of Arts and Sciences, Rachel Lindsey,
and Chairman of the Department of Biological Sciences, Dr Floyd Banks. Other supply
and maintenance funds come from director and major user grants as well as user fees.
For all Chicago State University members the De Novo Software Partner Program is offering
a 10% discount off on FCS Express Software. Please contact the Flow Cytometry facility
for details on how to receive the discount.
The Facs facility is supported by an NSF grant.
|
|
 The Herbarium in the Department of Biological Sciences at Chicago State University
was founded in 1989 by James R. Rastorfer, where it serves as a teaching and research
facility for students, faculty, and the university community. The Herbarium in the Department of Biological Sciences at Chicago State University
was founded in 1989 by James R. Rastorfer, where it serves as a teaching and research
facility for students, faculty, and the university community.
Our collection has specimens of lichens, bryophytes and vascular plants. However,
the largest group of plants represented in the collection are the flowering plants.
Particularly noteworthy in our collection of flowering plants are specimens from the
south side of Chicago and voucher specimens for collaborative land reclamation studies
with investigators at Argonne National Laboratory.
Contact information
Department of Biological Sciences / SCI 310
Chicago State University
9501 S. King Drive, Chicago, IL 60628
(773) 995-2183 voice
(773) 995-3759 fax
Email jgana@csu.edu
|
|
Mission Statement
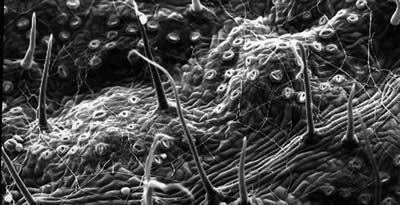
The Mission of the Chicago State University Central Microscopy Facility is to provide
the student researchers and faculty members with Teaching / Training, Access, Technical
Support and Service for a broad range of microscopy techniques.
Teaching / Training
The facility is used to teach Biology 4450 and 5450, Techniques in Electron Microscopy,
every other year. Further exposure to EM techniques in the form of workshops is offered
in the summer to students participating in several NIH funded summer programs. During
the academic year, the electron microscope is also available to the biology and chemistry
departments to support classroom activities. In years when the formal course is not
offered, individual training is available to assist students in integrating microscopy
techniques into their projects.
Access
The goal of the facility is the provide access to advance imaging instrumentation
to the CSU research community in an environment that fosters innovation and creativity.
A key part of this mission is to offer exposure to advance imaging technology to the
broadest cross section of students interested in STEM fields.
Technical Support
The microscopy facility aims to support faculty and student driven research in areas
of biology, chemistry, and physics by providing expertise in planning experiments,
collecting images and preparing images for publication. The facility also provides
a limited amount of hands-on support for faculty research projects.
Service
Microscopy education is another key part of the facility's mission. The facility provides
year round tours to prospective students from various Chicago land high schools, introductory
biology classes, and community groups. The facility is committed to seeking opportunities
to support faculty based community outreach and education efforts.
Facility Instruments
EM Microscopes
- JEOL JEM 1200EX Transmission Electron Microscope
Transmission electron microscopy allows high resolution imaging of thin sections of
biological material and negatively stained small particles. Electron diffraction of
crystalline samples can give information about the spacing of the atoms in the sample's
crystalline lattice. Images from this instrument are captured with a high resolution
digital camera.
The purchase of high resolution camera was funded by a Major Instrumentation Grant
from the National Science Foundation (NSF).
- JEOL JSM 6610LV Scanning Electron Microscope
SEM allows the observation of the surface details of a sample. This gives a magnification
range of 5x to 300,000x . This microscope is equipped with four detectors that provide
images of the surface topography of a sample and its elemental composition.
The design includes a low vacuum setting for samples that have excessive water content
or a non-conductive surface and cannot be viewed at high vacuum. This microscope is
equipped with a cold stage, which further extends its ability to look at hydrated
(wet) samples.
- Zeiss Axiovert 25 inverted Fluorescence Microscope with a Retiga CCD camera
The newest addition to the central facility is a Zeiss Axiovert 25 inverted fluorescence
light microscope with a cooled CCD digital camera and computer for image analysis.
This is a very flexible system that allows users to examine a wide range of samples
including live cells. The system also allows users to make time lapse movies.
- Zeiss Axioscope 20
- CR-X Cryosectioning System
- Epson 4990 Photo flatbed scanner and basic digital darkroom
- Image analysis software
- Classic wet darkroom facility
- various preparatory equipment for SEM
- Sputter Coater
Courses Offered at the Facility
Bio 4450 Techniques in Electron Microscopy Lecture AND Laboratory
Instrument and specimen preparation theory for both transmission TEM) and scanning
(SEM) electron microscopy. Preparation of specimens (ultramicrotomy, critical point
drying, negative staining) for examination in both TEM and SEM. Basic dark room and
digital image presentation. Substantial work outside of class time is required. Credit
will not be given for 4450 and 5450.
Bio 5450 Techniques in Electron Microscopy Lecture AND Laboratory
Instrument and specimen preparation theory for both transmission TEM) and scanning
(SEM) electron microscopy. Preparation of specimens (ultramicrotomy, critical point
drying, negative staining, X-ray analysis) for examination in both TEM and SEM. Fundamentals
of X-ray microanalysis and basic dark room and digital image presentation. Substantial
work outside of class time is required. Credit will not be given for 5450 and 4450.
If you are interested in taking a class please contact Dr. Maselli for details on
the next available section. (773) 995-3298
Publications & Presentations
- Reyes JF, Stone K, Ramos J, Maselli A. (2009) "Formation of Hirano bodies after inducible
expression of a modified form of an actin-cross-linking protein." Eukaryot Cell 8:852-7.
- The Ultrastructure Of The Parasitophorous Vacuole Formed by Leishmania Major. Castro,
R., Scott, K., Jordan, T., Evans, B., Craig, J., Peters, E. L., Swier, K. (2006).
Journal of Parasitology.
- Synthesis and characterization of a copper based dye sensitized solar cell. Moore,
Q. L.; Mardis, K. L.; LeSuer, R. J. National American Chemical Society meeting, San
Francisco, 2010.
- Investigating the impact of titanium dioxide slurry preparation on the performance
of dye sensitized solar cells. Timmons, A.; LeSuer, R. J. National American Chemical
Society meeting, San Francisco, 2010.
- Towards understanding electron transfer processes in dye sensitized solar cells. LeSuer,
R. J. Center for Alternative Energy and Technology Symposium, Chicago, 2009.
- Patrick, D., Maselli, A. Cells Expressing a Predicted Caspase I Cleavage Product of
T-plastin Produce Actin Inclusions, The American Society of Cell Biology 48th annual
meeting. 2008 (Presented by A. Maselli).
- Maselli, A., Ramos, J., Patrick, D. Hwang, R., Knecht, D. (2008). Comparison of the
Properties of Actin Aggregates Induced by Fragments of Different Actin Binding Proteins.
International Dictyostelium Conference, Tsukuba-Shi, Japan.
- Ramos, J., M. Myrthil, K. Stone, A, Maselli, Comparison of Hirano Body Fromation by
an F-Actin Stabilizing Drug and A Truncated Acting Bundling Protein. Illinois State
Academy of Sciences Annual Meeting 2007.
- J. Ramos, A. Maselli (2006). Exploring Drug Stabilized F-actin as a Live Cell Model
for Hirano Body Formation. The American Society of Cell Biology 46th annual meeting.
- The Formation of GFP-Tagged Hirano Bodies in Dictyostelium discoideum After Inducible
Expression of a Truncated Actin Bundling Protein. J. F. Reyes, A. G. Maselli. The
American Society of Cell Biology 45th annual meeting. 2006
- Dictyostelium cells with GFP tagged Hirano bodies grow more slowely than wild type.
M. Myrthil, J. F. Reyes, and A. Maselli. Chicago State University. Illinois State
Academy of Science Annual Meeting. 2006
- Promoter inducible response of RootCAR1, a cold acclimation response gene in alfalfa
(Medicago sativa L.) using protoplast transient assays. Singa P, Gana JA Midwest American
Society of Plant Biologists Sectional Meeting. March 18-19. St. Louis, MO *. 2005
- Comparison of amyloplast structure and numbers in mature intact and defoliated alfalfa
plants (Medicago sativa L.) by transmission electron microscopy. Yosimbom E, Gana
JA Midwest American Society of Plant Biologists Sectional Meeting. March 18-19. St.
Louis, MO* 2005
- Isolation of a new class of Vancomycin Resistent Enterococci from Raw Minced Pork.
Park, G.B., Sinja, R. American Society of Microbiologists. Atlanta GA. 2005
- Correlation between internal structure and macromolecule accumulation in two cultivars
of sweet potato under salt stress. Weathington, T.O., Marshal, A.K., Potluri, V. Illinois
Student Research Conference. Charleston IL. 2006.
Contact Information
 Andrew G. Maselli Andrew G. Maselli
Facility Director
Department of Biological Sciences
Chicago State University
9501 S. King Drive/ SCI 310
Chicago, IL 60628
(773) 995-3298 voice
(773) 995-3759 fax
|
|
Chicago State University is part of a wider community dedicated to helping preserve
one of North America’s most imperiled ecosystems, the Tallgrass Prairie. A prairie
is a landscape dominated by grasses, yet contains numerous flowering plants. In 1820,
Illinois contained 22 million acres of prairie along with 14 million acres of forest,
leading to the state nickname of “The Prairie State.” However, by the early 1900’s,
most prairies had been plowed for agriculture and were destroyed. In Illinois today,
there is less than 2,500 acres remaining, or 0.01% of the original prairie landscape.
Did you know that the world grasslands, including the North American prairie, are
the most endangered ecosystem in the world? In the United States and Canada, 99%
of the prairie is gone. The remaining original prairie, called remnants, exists in
small pockets, and are often under a few acres in size, leading to the loss of plant,
animal and insect species.
Today, Chicago State University is helping reverse this trend by planting native prairie
plants in its 3.5-acre prairie garden and bird habitat. The over 80 native grasses,
flowering plants, trees and shrubs provide the habitat needed for native butterflies
(including monarchs) and birds. These plants also help the community by removing pollutants
from the air and by utilizing their deep root systems, flooding is reduced because
native plants absorb more water than turf grass.
History of the Prairie Garden
In 2003, Dr. Timothy Bell received an Illinois Department of Natural Resources (IDNR)
grant to install the prairie garden on campus. Dr. Potluri obtained a second IDNR
grant and appointed a volunteer coordinator Ms. Kelly Borger who entered the prairie
garden in the citywide competition. Since this time, through the help of volunteers,
students and additional grants, a bird habitat was created, a compost station for
weeds was installed, as were bat and bluebird houses. Volunteers work to maintain
the path system, remove weedy plants that do not belong in the prairie, collect seeds,
grow native plants in the greenhouse and plant them in the prairie. Students have
also conducted research on the soil, plants, and insects that are using the prairie.
Grants and Partners
Illinois Department of Natural Resources
Illinois Louis Stokes Alliance for Minority Participation (LSAMP) Urban Science, Technology,
Engineering, Mathematics Talent Expansion Program (USTEP)
Contact Information
Department of Biological Sciences
Phone 771-995-2183
jgana@csu.edu
|


 Andrew G. Maselli
Andrew G. Maselli
 All Rights Reserved
All Rights Reserved